5.0 ★★★★★
2326 Reviews
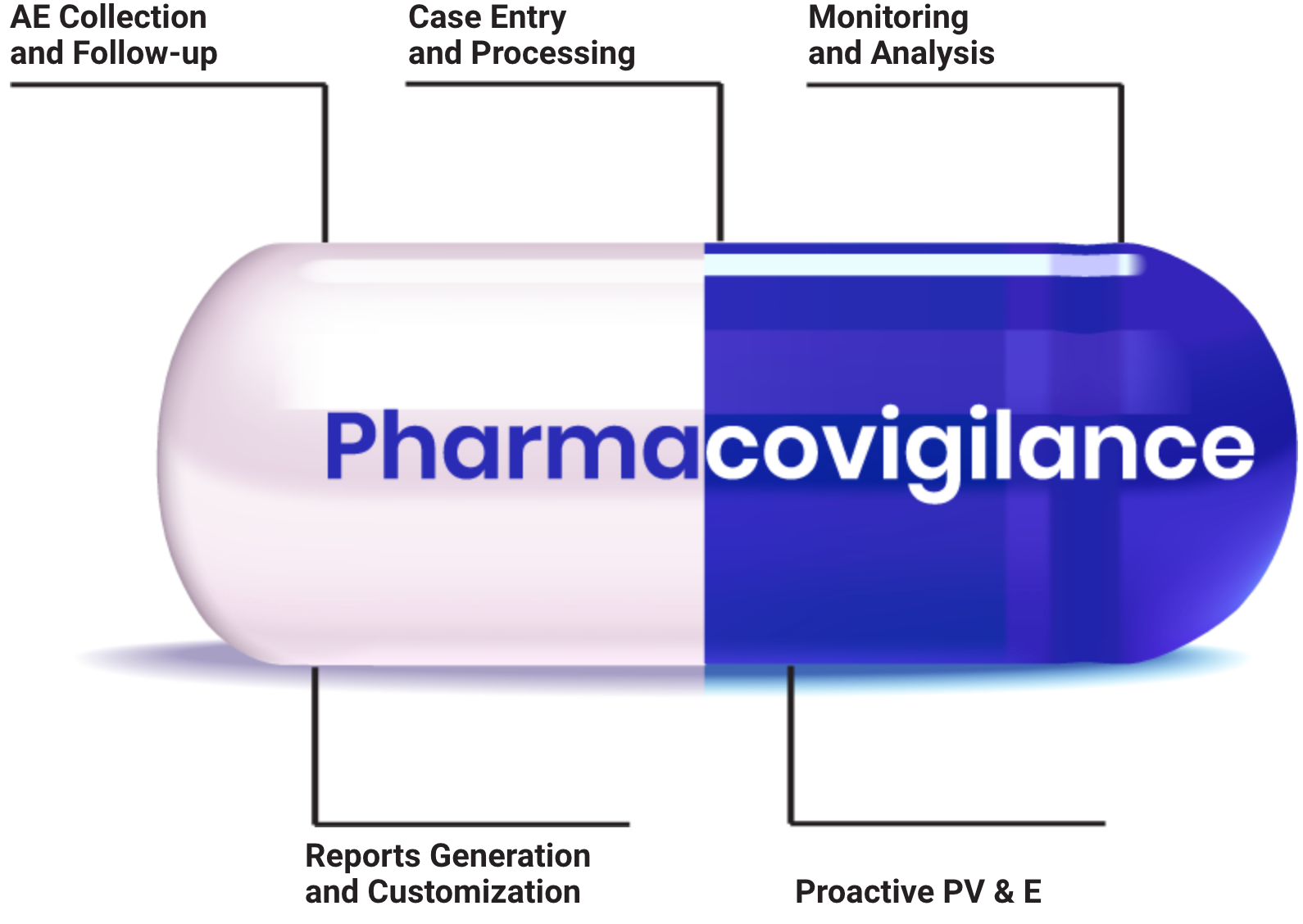
Best Pharmacovigilance Training
 Master drug safety monitoring protocols
Master drug safety monitoring protocols Learn adverse event reporting systems
Learn adverse event reporting systems Understand regulatory compliance requirements
Understand regulatory compliance requirements Develop risk assessment skills
Develop risk assessment skills Gain pharmaceutical industry knowledge
Gain pharmaceutical industry knowledge